Abstract
Introduction
The transfer of patients with severe acute respiratory distress syndrome (ARDS) to extracorporeal membrane oxygenation (ECMO) capable centers is a prevalent practice, despite a paucity of data regarding the outcome of transferred patients and those remaining in a community setting with standardized care. Our aim was to compare outcomes in these two patient populations.
Methods
Adult patients admitted to a community health system with SARS-CoV-2 infection requiring invasive mechanical ventilation (IMV) from February 2020 to July 2022 were identified. We performed univariate and multivariable logistic regression (adjusting for demographics and severity of illness). Categorical data are presented as percentages, and continuous data as median [25-75% Interquartile range].
Results
397 patients were identified with COVID-19 requiring IMV, and 29 were transferred to the ECMO center. Non-transferred patients were older, 64 [56-73] vs. 48 [40-55] (p < 0.001), with a higher proportion of comorbid conditions. Both groups had similar initial P/F ratios, trending towards a lower P/F in the transferred group at 24 hours after IMV: 121 [88-167] vs. 105 [75-132] (p = 0.083). The organ-specific (renal, liver, coagulation) SOFA score was lower in transferred patients, 0 [0-1] vs. 1 [0-2] (p = 0.007). Two hundred eighty-two patients (71.2%) in the community health system died. Among those transferred, 21 (72.4%) were not supported with ECMO, and 9 (42.9%) died. Of the 8 patients supported with ECMO, 3 (37.5%) died. Transfer to a tertiary care center was associated with a reduced risk of death (OR 0.25; 95% CI 0.11-0.55, p = 0.001), which persisted when adjusted for age, P/F ratio, & organ-specific SOFA scores (OR 0.34; 95% CI 0.15-0.80, p = 0.013).
Conclusions
Patients with severe ARDS have a reduction in mortality when transferred to an ECMO-capable center despite providing similar care in both settings. An emphasis on appropriate patient selection is important, specifically focusing on ECMO-eligible patients. More research is needed to identify the variables impacting mortality in these patient populations.
Introduction
Acute respiratory distress syndrome (ARDS) is a life-threatening inflammatory lung condition that severe coronavirus-19 (COVID-19) frequently causes [1-3]. Prior literature recommends transferring patients with severe ARDS to centers specializing in ARDS management [4]. During the COVID-19 pandemic, the capacity of such centers was exceeded by the patient volume [5,6], resulting in centers previously less experienced in ARDS management and treatment to adapt and care for these patients. Fortunately, effective medical management for patients with ARDS is established, with the hallmarks including lung protective ventilation, prone positioning, and maintenance of euvolemia. In contrast, guidelines regarding the management of patients with COVID-induced ARDS were evolving during this period. In addition to appropriate standard ARDS care, recommendations for the use of corticosteroids, baricitinib, and tocilizumab were variable.
Data has shown that transferring patients with severe ARDS, whether on or off ECMO, carries risk [7,8]. Meanwhile, the CESAR trial supports transferring patients with severe ARDS to ECMO-capable centers.4 No study has compared patient outcomes at ECMO-capable centers versus non-ECMO-capable centers when the same group of physicians cares for both groups of patients. One important variable when potentially comparing both populations is that, in many instances, there is a different group of physicians with different practice patterns, protocols, and ventilator management strategies that could eventually account for potential differences. In our study, patients were managed in either setting by one ICU group with standardized practice. Given this, we hypothesize there would be no difference in mortality between the groups.
Methods
Design
We conducted a retrospective observational cohort study of outcomes of patients who were cared for entirely at a community hospital system (Inspira Health Network) to patients who were cared for initially at the same community hospital and then transferred to one tertiary care ECMO referral center (Cooper University Hospital) in New Jersey, USA.
The community health system consists of 4 hospitals. Two of those hospitals have ICUs that care for patients with ARDS. They have a combined 410 total beds, including 56 ICU beds. It is staffed by board-certified critical care physicians employed by Cooper University Healthcare with 24-hour critical care fellow coverage. The referral hospital is a 620-bed, level 1 trauma center with 100 ICU beds and a 2:1 nursing ratio. All the ICUs were staffed by the same intensivist group with standardized practice patterns as far as medication use, ventilator management, prone positioning, paralytic use, and threshold for ECMO referral. At the receiving center, VV ECMO cannulation was considered when one of the following criteria was met PaO2/FiO2 < 80 mmHg after optimal medical management, including a trial of prone positioning or hypercapnic respiratory failure (pH <7.25), despite optimal conventional mechanical ventilation (respiratory rate 35 bpm and plateau pressure<30 cmH2O) in the absence of any contraindications. The major contraindications included a moribund state in which ECMO is unlikely to change the outcome, prolonged mechanical ventilation, or severe chronic comorbidities that may interfere with recovery. The center performed between 15 and 20 ECMO cases annually pre-COVID-19 pandemic, which doubled during the COVID-19 pandemic. VV ECMO survival during the study years was 59.1%.
Patients, data, outcomes
We included patients aged 18 years and older with SARS-CoV-2 infection (diagnosed by nasal PCR) requiring invasive mechanical ventilation (IMV) at the community health system between February 2020 and July 2022. The data were manually extracted using the electronic medical record system at each institution. The study was approved by the Institutional Review Board at Inspira Health (2022-07-001) and Cooper University Healthcare (22-118). The requirement for informed consent was waived.
Demographics, baseline characteristics, comorbidities, duration of various interventions, therapies received, and transfer status were collected. Time was defined in “days” beginning with the day of initiation of IMV. The primary outcome of interest was in-hospital mortality.
Statistical analysis
The distribution of all continuous data was examined for normality using visual inspection and the Wilk-Shapiro test. Characteristics of the groups are presented as the median and 25-75% interquartile range (IQR) and compared using the Wilcoxon rank-sum test. Categorical data are presented as counts with proportions and compared using Fisher’s exact test. In-hospital mortality between patients referred to an ECMO center and those who were not was assessed through multivariable logistic regression. The models were adjusted based on factors selected a priori, given the known association with ECMO use and hospital mortality (age, PaO2/FiO2 [P/F] ratio, and organ-specific SOFA score).
As patients transferred to an ECMO center were anticipated to have spent time at the primary center after initiation of IMV, the possibility that patients in the non-referral group died prior to the feasibility of referral to an ECMO center was considered an important confounding factor. To minimize the effect of this bias, outcomes were compared primarily between patients who survived at least as long as the median duration of IMV (> 1 day) prior to transfer among patients referred to the tertiary care center. Likewise, patients designated for a “comfort measures only” [hospice] care strategy prior to this median duration were excluded from the primary analysis.
Two pre-specified sensitivity analyses were also performed. First, in-hospital mortality was compared between patients transferred to the tertiary care center and those who were not utilizing propensity score matching based on age, P/F ratio, and organ-specific SOFA score. Propensity score matching was performed using a 1:1 nearest neighbor matching algorithm with a caliper of 0.2. Patients who survived for at least one day after requiring IMV were included in this analysis. Second, the primary outcome was reanalyzed, excluding any patient who died or was placed on hospice within 2 days of initiation of IMV. No a priori statistical power calculation was conducted. All relevant statistical tests were two-tailed, and a p-value < 0.05 was considered statistically significant. All statistical analyses were performed using STATA version 14 (StataCorp LP; College Station, TX).
Results
Patient characteristics
A total of 397 patients with COVID-19 required IMV within the community health system during the study period. Thirty patients (7.6%) were referred and transferred to the tertiary care facility for consideration of ECMO support for severe ARDS. One patient was transferred to an outside hospital system where follow-up data was not available, and this patient was excluded from further analysis. The characteristics of the 396 patients are presented in Table 1. Among all patients, the median age was 63 years [54-73], 178 (45.0%) were female, and the median BMI was 32.7 kg/m2 [27.6-38.4]. Most patients, 60.9% and 62.3%, were supported with high-flow nasal cannula (HFNC) and/or non-invasive ventilation (NIV; continuous positive pressure airway support [CPAP] or bilevel positive pressure airway support [BPAP]) prior to endotracheal intubation, respectively. The median duration of respiratory support (HFNC and NIV) prior to endotracheal intubation was 7 days [2-15], and the initial P/F ratio following initiation of IMV was 82 [66-121]. In total, 282 patients (71.2%) who required IMV at the community health system died. Among patients treated entirely at the community health system, comorbidities such as COPD, hypertension, and diabetes mellitus were all significantly more common compared to those patients transferred to the tertiary care facility. The median duration of mechanical ventilation among those managed solely at the community center was 10 days [5-17].
Among the patients referred to the tertiary care center, 21 (72.4%) ultimately were never supported with ECMO. Of those transferred but not supported with ECMO, 9 (42.9%) died. Of the 8 patients supported with ECMO, 3 (37.5%) died.
Clinical care during hospitalization
Administration of Tocilizumab was less common in patients not referred to the tertiary center (Table 2). 141 patients were converted to a hospice with N=28 and N=47 of these changes made within 1 or 2 days of endotracheal intubation, respectively. In total, 270 (73.6%) of patients managed exclusively at the community health system died.
Mortality outcome
Within the entire cohort, transfer to the tertiary care center was significantly associated with a reduced risk of death (OR: 0.25; 95% CI: 0.11-0.55, p-value=0.001). This finding persisted after the model was adjusted for age, P/F ratio, and organ-specific SOFA score (OR: 0.34; 95% CI: 0.15-0.80, p-value=0.013) (Table 3). A comparison of comorbidities, illness severity, clinical care, and outcomes among patients after the exclusion of those who did not survive at least one day of IMV is provided in sTable 1 and 2. Despite the exclusion of these patients, the risk of death remained significantly lower in patients transferred to the tertiary care center (OR: 0.28; 95% CI: 0.13-0.61, p-value = 0.001 and adj OR: 0.35; 95% CI: 0.15, 0.82, p-value = 0.015) (sTable 3).
Finally, two pre-specified sensitivity analyses were performed to evaluate the robustness of the above findings. First, propensity score matching based on age, P/F ratio, and organ-specific SOFA score again demonstrated a reduced risk of death among patients transferred to the tertiary care compared to those that remained at the referral center (OR: 0.71; 95% CI: 0.56-0.91, p-value=0.006). Second, the finding of reduced risk of death in the transfer group persisted after the primary analysis was repeated after the exclusion of the 47 patients at the community health system that did not survive for at least 2 days after initiation of IMV (OR: 0.29; 95% CI: 0.13-0.63, p-value = 0.002 and adj OR: 0.26; 95% CI: 0.08-0.83, p-value = 0.023).
Discussion
In this retrospective cohort study examining patients with COVID-19 ARDS treated by one intensivist group in both the community center and those transferred to an ECMO referral center, mortality was significantly reduced in the group that was transferred. While the practices in both institutions are similar, with the same intensivist group staffing the ICUs, a selection bias likely exists as the patients with increased survivability were probably more likely to be transferred. Our results may be quantifying the differences in care provided by consultant services, as well as varying nursing and respiratory therapy protocols.
The care of patients with ARDS is rooted in varying degrees of evidence. Best practices include the use of lung protective ventilation (target tidal volume 6-8 cc/kg ideal body weight) [9-11], positive end-expiratory pressure (PEEP) optimization to minimize barotrauma (PPlat < 30 cmH2O), and improve static lung compliance (driving pressure < 14 cmH2O) [12,13], as well as a conservative fluid management strategy [14,15]. The timely implementation of prone ventilation in those with severe disease[16], as defined by a P/F < 100, likely has the strongest evidence, as seen in a significant mortality benefit [17,18]. Other practices, such as the use of inhaled pulmonary vasodilators [19,20], neuromuscular blockade [21-23], and extracorporeal mechanical oxygenation [24-27], while frequently utilized in the care of these patients, have less robust evidence to support their use.
The CESAR trial, designed to compare conventional mechanical ventilatory care to the use of ECMO in patients with severe ARDS, did not find a statistically significant reduction of mortality in the ECMO group [4]. However, in those transferred to a hospital specialized in ARDS (i.e., an ECMO-capable center), there was a reduction in mortality when compared with those patients who remained at the transferring center, regardless of whether the patient underwent ECMO. Several important flaws exist in the CESAR trial’s design. Only 75% of the ECMO group received mechanical support, and, most importantly, the care of the control group was not standardized. More specifically, the trial did not control for the approach to lung protective ventilation, nor did it specify the indication for prone-ventilation. These two interventions have been shown to be crucial in caring for patients with ARDS. However, the author’s conclusion that those with severe ARDS should be transferred to a hospital with ECMO capabilities remains frequently cited. A follow-up to this study examined outcomes in adult patients admitted with ARDS between 2010 and 2016 (n = 224,447), comparing ECMO versus non-ECMO centers. Adjusted results showed no difference in mortality (OR 0.99, 95% CI: 0.97, 1.02) [28].
It is important to highlight that the care of patients with severe ARDS has become more sophisticated since the CESAR trial was published. Tools designed to aid in advanced respiratory monitoring allow for management adjustments that may have a positive impact on outcomes. These include esophageal pressure monitoring, which provides an estimate of pleural pressure allowing for PEEP optimization, measurement of the electrical activity of the diaphragm leading to minimization of patient-ventilator dyssynchrony, electrical impedance tomography, which provides a way to minimize airway overdistention, and ultrasound assessment of lung and respiratory muscles, which has a multitude of benefits [29]. This specialized care may be even more important in patients on VV ECMO [30]. Centers that provide ECMO care are more likely to be capable of incorporating these advanced tools into the care of ARDS patients, likely adding to improved outcomes. Interestingly, these advanced technologies were not available at the ECMO-capable center during the study period, and their impact on clinical outcomes in ARDS remains an important area of future research.
Comparing outcomes between community and referral centers can be difficult due to differences in physicians' practices at each site. This study provided a unique chance to control for this common confounding factor—practice pattern variations, since the same physicians treated patients in both settings. Assuming disease severity was accounted for, we hypothesized there would be no difference in outcomes.
The hypothesis was not supported; mortality in patients transferred was significantly lower than those who remained in the community hospital (unadjusted OR: 0.25, 95% CI: 0.11-0.55, p-value < 0.001). This alone is not surprising, as prior to transfer, mandatory caveats are met: first, the patient would benefit from transfer, implying they do not have any absolute contraindications to ECMO, and second, the patient is able to be transferred safely and is not moribund. To control for this selection bias, pre-determined influential variables were selected. The mortality benefit persisted despite adjustment for age, PaO2/FiO2 ratio, and SOFA score (OR: 0.34, 95% CI 0.15-0.8, p-value = 0.013). If patients who die or are transitioned to hospice within 2 days of intubation are removed, the relationship remained unchanged (adj OR: 0.26; 95% CI: 0.08-0.8, p-value = 0.023). These results imply there may be a mortality benefit to transferring patients with ARDS to an ECMO-capable center. Of the transferred patients, 8 were cannulated for ECMO, with 5 surviving to discharge. This may have impacted the primary outcome, and meaningful conclusions regarding the impact of receipt of ECMO at the tertiary center from this limited data are not possible. However, multiple potential explanations for the observed mortality benefit of transfer can be offered
First, the care of patients with ARDS requires a multifaceted approach. Applying evidence-based clinical care alone is not enough. Nursing and ancillary staff expertise play a major role in outcome determination. Examples such as head turning while in a prone position, titration of the paralytic to the minimal effective dose, and recognition of ventilator dyssynchrony require experience.
Similarly, despite having the same attending physicians, the staffing model differed between the two settings. The presence of an attending physician 24 hours a day, as compared to 8-hour coverage with an in-house critical care fellow and remote attending coverage to fill in the gaps, could contribute to the difference in outcomes. The addition of constant attending coverage may allow for expedited advancement of care (for example, the timely use of prone ventilation and off-hour ventilator liberation), as well as expert evaluation of unanticipated complications. As an example, endotracheal intubation carries significant morbidity, especially in those with low reserve or significant hypoxia; patients with ARDS certainly qualify as these. This can be decreased by experienced providers performing the procedure [31]. The clinical benefit of 24-hour intensivist coverage is not novel [32]. Similarly, large academic centers offer increased availability of other consulting services, including surgical subspecialists, in the event that complications arise. This could provide further undetected patient benefits.
A third explanation is that other covariates that are not controlled for are influencing the results. To minimize this, a variety of clinical factors were collected. There was no significant difference between the groups in chronic comorbidities, except for hypertension, respiratory support pre-intubation, which has been linked to lung injury and poorer outcomes [33], and COVID-19-specific therapeutics utilized. However, other differences certainly exist, such as COVID-19 vaccination status and the wave of the COVID-19 pandemic. The ability to create a more robust model was limited due to the sample size. Specifically, the group of transferred patients was small.
Finally, a confounding factor is likely that the patients transferred were those with a potentially higher chance of survival. This is evident by the younger age of the cohort of patients transferred to the tertiary care center. These patients are also likely to have a longer length of stay with a need for tracheostomy. Along with this, there would be a greater potential for barotrauma.
This study has limitations. The knowledge of appropriate management does not guarantee compliance with these guidelines. The collection of clinical variables to ensure appropriate care was provided would have increased the reliability of the results. In addition, other variables and clinical data could have been collected to show that the comparison groups are similar. For example, clinical prediction scores related to the severity of critical illness, ARDS, and the probability of survival with ECMO support were not collected. Likewise, the administration of corticosteroids as part of the treatment of COVID-19 was similarly not evaluated. Likely, corticosteroids were utilized at similar rates between the two institutions, but this missing data reflects an important limitation of our analysis. The low sample size of the transfer group and unavoidable selection bias also make the generalizability of these results less feasible. Though attempts were made to correct for covariates, all potentially important confounders could not be considered, and residual confounding is possible. The high percentage of patients supported by ECMO could be seen as a weakness. However, given the expanding ability for off-site ECMO cannulation and the growth of the number of ECMO-capable centers, this treatment approach should be in the arsenal of all ICUs, even if the center itself does not have an ECMO program. A large previous study has demonstrated similar results reflective of improved survival among patients cared for at an ECMO referral center [4]. Our results reaffirm this prior finding and additionally demonstrate improved survival despite standardized clinical practices at the two included institutions.
Conclusions
Ultimately, this study highlights the improved survivability in younger, baseline healthier patients with ARDS and demonstrates the multifaceted approach needed to treat these patients. The transfer of eligible patients who may benefit from the care of ECMO-capable centers is still supported.
Tables and Figures
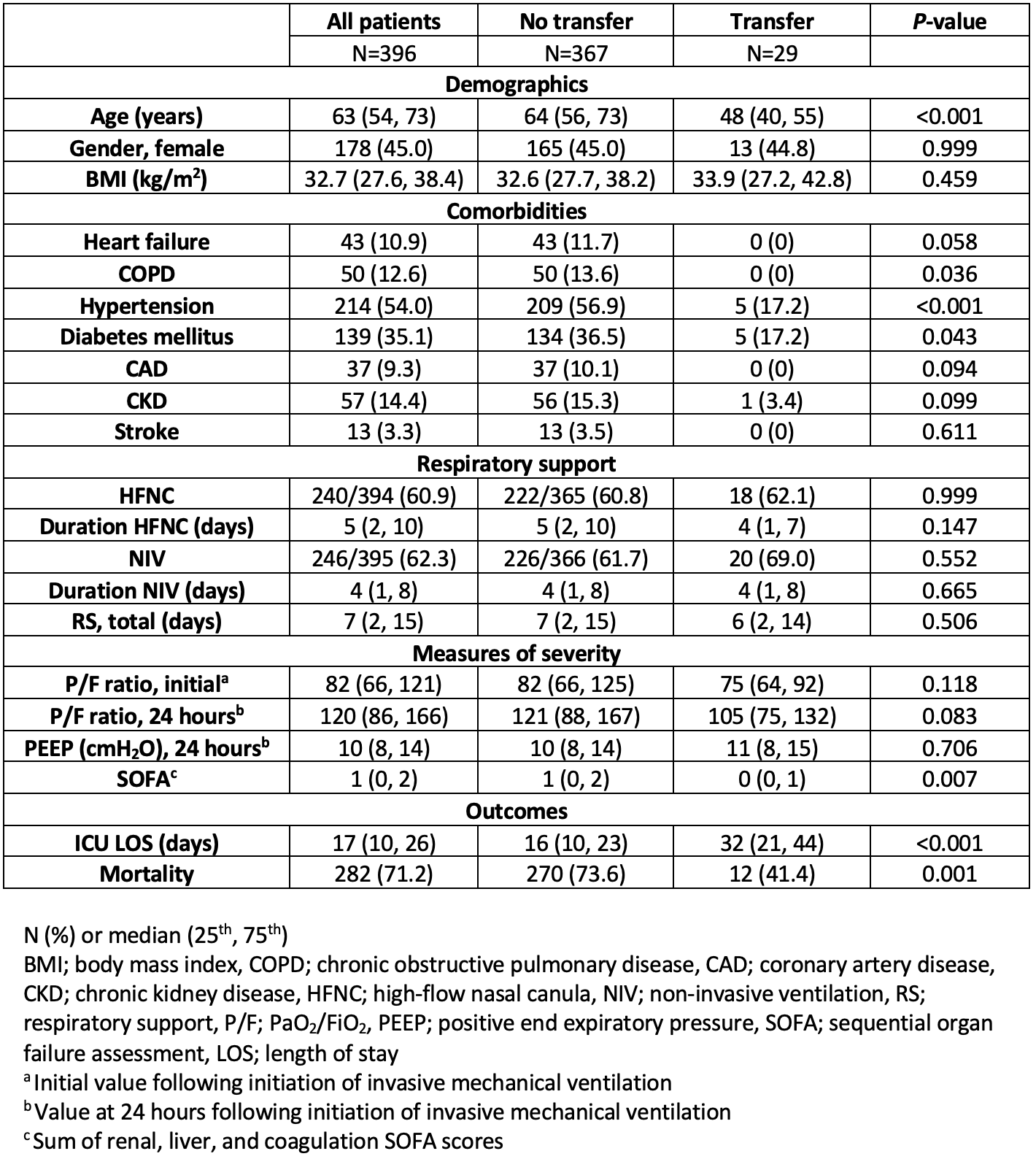
Table 1. Univariate analysis of patients based on transfer status.
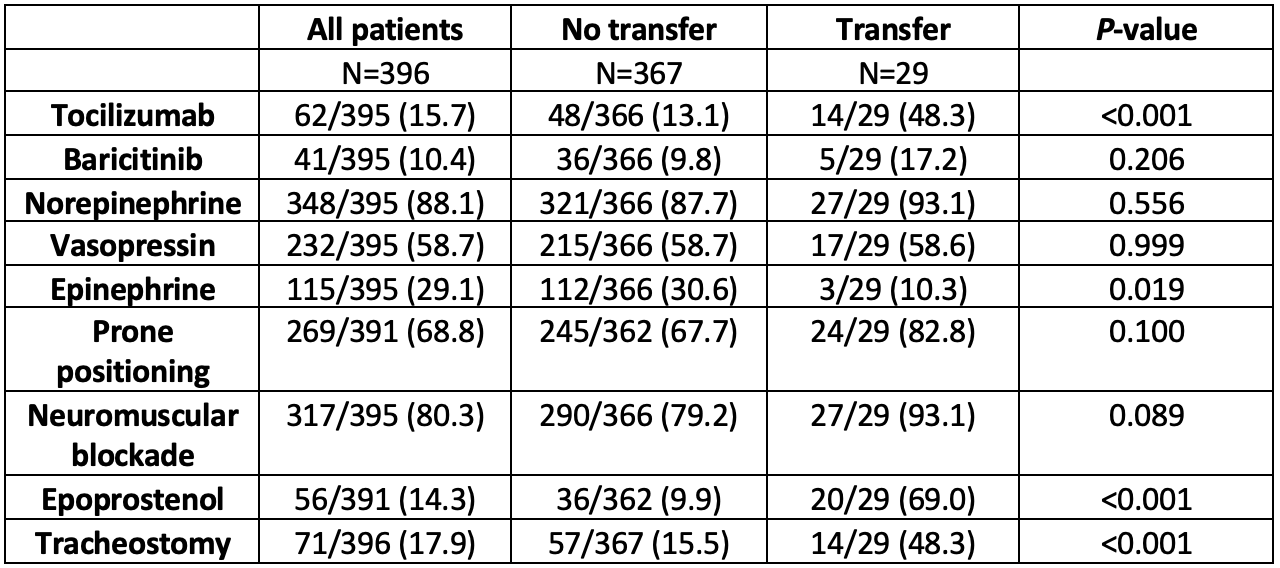
Table 2. Clinical care received during the duration of hospitalization.
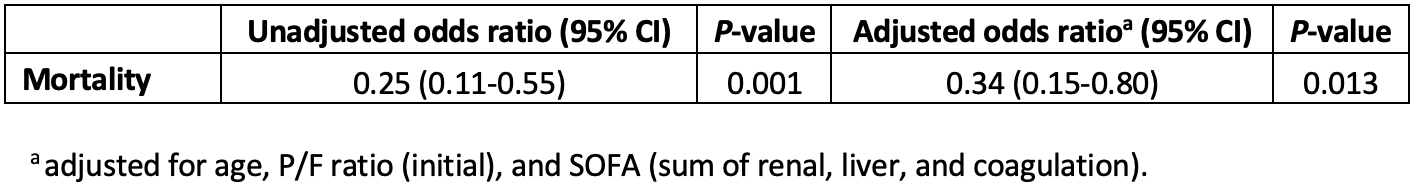
Table 3. Risk of death stratified by transfer status (No transfer as reference) with adjustment for confounders (all patients).
Details
Acknowledgements
None.
Disclosures
None.
Data Availability Statement
Data is available upon appropriate request.
Supplementary Files
References
- 1
Tzotzos SJ, Fischer B, Fischer H, Zeitlinger M. Incidence of ARDS and outcomes in hospitalized patients with COVID-19: a global literature survey. Critical Care. 2020 Aug 21;24(1):516.
- 2
Gupta S, Hayek SS, Wang W, Chan L, Mathews KS, Melamed ML, Brenner SK, Leonberg-Yoo A, Schenck EJ, Radbel J, Reiser J. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Internal Medicine. 2020 Nov 1;180(11):1436-47.
- 3
Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. American journal of respiratory and critical care medicine. 1994 Mar;149(3):818-24.
- 4
Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, Hibbert CL, Truesdale A, Clemens F, Cooper N, Firmin RK. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. The Lancet. 2009 Oct 17;374(9698):1351-63.
- 5
Duggal A, Orsini E, Mireles-Cabodevila E, Krishnan S, Rajendram P, Carpenter R, Khouli H, Hatipoglu U, Dweik R. Surge capacity and capability of intensive care units across a large healthcare system: An operational blueprint for regional integration. American journal of disaster medicine. 2021 Sep 1;16(3):179-92.
- 6
Tyrrell CS, Mytton OT, Gentry SV, Thomas-Meyer M, Allen JL, Narula AA, McGrath B, Lupton M, Broadbent J, Ahmed A, Mavrodaris A. Managing intensive care admissions when there are not enough beds during the COVID-19 pandemic: a systematic review. Thorax. 2021 Mar 1;76(3):302-12.
- 7
Blecha S, Dodoo-Schittko F, Brandstetter S, Brandl M, Dittmar M, Graf BM, Karagiannidis C, Apfelbacher C, Bein T. Quality of inter-hospital transportation in 431 transport survivor patients suffering from acute respiratory distress syndrome referred to specialist centers. Annals of Intensive Care. 2018 Jan 15;8(1):5.
- 8
Linden V, Palmer K, Reinhard J, Westman R, Ehren H, Granholm T, Frenckner B. Inter-hospital transportation of patients with severe acute respiratory failure on extracorporeal membrane oxygenation–national and international experience. Intensive care medicine. 2001 Oct;27(10):1643-8.
- 9
Petrucci N, Iacovelli W. Lung protective ventilation strategy for the acute respiratory distress syndrome. Cochrane Database of Systematic Reviews. 2007(3).
- 10
Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. New England Journal of Medicine. 2000 May 4;342(18):1301-8.
- 11
Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, Takagaki TY. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. New England Journal of Medicine. 1998 Feb 5;338(6):347-54.
- 12
Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, Stewart TE, Briel M, Talmor D, Mercat A, Richard JC. Driving pressure and survival in the acute respiratory distress syndrome. New England Journal of Medicine. 2015 Feb 19;372(8):747-55.
- 13
Mercat A, Richard JC, Vielle B, Jaber S, Osman D, Diehl JL, Lefrant JY, Prat G, Richecoeur J, Nieszkowska A, Gervais C. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. Jama. 2008 Feb 13;299(6):646-55.
- 14
Silversides JA, Major E, Ferguson AJ, Mann EE, McAuley DF, Marshall JC, Blackwood B, Fan E. Conservative fluid management or deresuscitation for patients with sepsis or acute respiratory distress syndrome following the resuscitation phase of critical illness: a systematic review and meta-analysis. Intensive care medicine. 2017 Feb;43(2):155-70.
- 15
Yang CS, Liu Y, Lu XM, Liu SQ, Guo FM, Yang Y, Qiu HB. Effect of conservative fluid management strategy on the outcomes in patients with acute lung injury: a meta-analysis. Zhonghua yi xue za zhi. 2011 Jun 1;91(21):1471-4.
- 16
Ferguson ND, Fan E, Camporota L, Antonelli M, Anzueto A, Beale R, Brochard L, Brower R, Esteban A, Gattinoni L, Rhodes A. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive care medicine. 2012 Oct;38(10):1573-82.
- 17
Beitler JR, Shaefi S, Montesi SB, Devlin A, Loring SH, Talmor D, Malhotra A. Prone positioning reduces mortality from acute respiratory distress syndrome in the low tidal volume era: a meta-analysis. Intensive care medicine. 2014 Mar;40(3):332-41.
- 18
Guérin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, Mercier E, Badet M, Mercat A, Baudin O, Clavel M. Prone positioning in severe acute respiratory distress syndrome. New England journal of medicine. 2013 Jun 6;368(23):2159-68.
- 19
Adhikari NK, Burns KE, Friedrich JO, Granton JT, Cook DJ, Meade MO. Effect of nitric oxide on oxygenation and mortality in acute lung injury: systematic review and meta-analysis. Bmj. 2007 Apr 12;334(7597):779.
- 20
Taylor RW, Zimmerman JL, Dellinger RP, Straube RC, Criner GJ, Davis Jr K, Kelly KM, Smith TC, Small RJ, Inhaled Nitric Oxide in ARDS Study Group, Inhaled Nitric Oxide in ARDS Study Group. Low-dose inhaled nitric oxide in patients with acute lung injury: a randomized controlled trial. Jama. 2004 Apr 7;291(13):1603-9.
- 21
Alhazzani W, Alshahrani M, Jaeschke R, Forel JM, Papazian L, Sevransky J, Meade MO. Neuromuscular blocking agents in acute respiratory distress syndrome: a systematic review and meta-analysis of randomized controlled trials. Critical care. 2013 Mar 11;17(2):R43.
- 22
Neto AS, Pereira VG, Espósito DC, Damasceno MC, Schultz MJ. Neuromuscular blocking agents in patients with acute respiratory distress syndrome: a summary of the current evidence from three randomized controlled trials. Annals of intensive care. 2012 Jul 26;2(1):33.
- 23
Papazian L, Forel JM, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, Jaber S, Arnal JM, Perez D, Seghboyan JM, Constantin JM. Neuromuscular blockers in early acute respiratory distress syndrome. New England Journal of Medicine. 2010 Sep 16;363(12):1107-16.
- 24
Munshi L, Telesnicki T, Walkey A, Fan E. Extracorporeal life support for acute respiratory failure. A systematic review and meta-analysis. Annals of the American Thoracic Society. 2014 Jun;11(5):802-10.
- 25
Zampieri FG, Mendes PV, Ranzani OT, Taniguchi LU, Azevedo LC, Costa EL, Park M. Extracorporeal membrane oxygenation for severe respiratory failure in adult patients: a systematic review and meta-analysis of current evidence. Journal of Critical Care. 2013 Dec 1;28(6):998-1005.
- 26
Peek GJ, Clemens F, Elbourne D, Firmin R, Hardy P, Hibbert C, Killer H, Mugford M, Thalanany M, Tiruvoipati R, Truesdale A. CESAR: conventional ventilatory support vs extracorporeal membrane oxygenation for severe adult respiratory failure. BMC Health Services Research. 2006 Dec 23;6(1):163.
- 27
Zapol WM, Snider MT, Hill JD, Fallat RJ, Bartlett RH, Edmunds LH, Morris AH, Peirce EC, Thomas AN, Proctor HJ, Drinker PA. Extracorporeal membrane oxygenation in severe acute respiratory failure: a randomized prospective study. Jama. 1979 Nov 16;242(20):2193-6.
- 28
Maine RG, Strassle P, Orleans B, Bryant MK, Raff L, Reid T, Charles A. Inpatient mortality among patients with acute respiratory distress syndrome at ECMO and non-ECMO centers in the United States. The American Surgeon™. 2023 May;89(5):1512-8.
- 29
Cammarota G, Simonte R, Longhini F, Spadaro S, Vetrugno L, De Robertis E. Advanced point-of-care bedside monitoring for acute respiratory failure. Anesthesiology. 2023 Feb 7;138(3):317-34.
- 30
Simonte R, Cammarota G, Vetrugno L, De Robertis E, Longhini F, Spadaro S. Advanced respiratory monitoring during extracorporeal membrane oxygenation. Journal of Clinical Medicine. 2024 Apr 26;13(9):2541.
- 31
Buis ML, Maissan IM, Hoeks SE, Klimek M, Stolker RJ. Defining the learning curve for endotracheal intubation using direct laryngoscopy: a systematic review. Resuscitation. 2016 Feb 1;99:63-71.
- 32
Wilcox ME, Chong CA, Niven DJ, Rubenfeld GD, Rowan KM, Wunsch H, Fan E. Do intensivist staffing patterns influence hospital mortality following ICU admission? A systematic review and meta-analysis. Critical care medicine. 2013 Oct 1;41(10):2253-74.
- 33
Battaglini D, Robba C, Ball L, Silva PL, Cruz FF, Pelosi P, Rocco PR. Noninvasive respiratory support and patient self-inflicted lung injury in COVID-19: a narrative review. British Journal of Anaesthesia. 2021 Sep 1;127(3):353-64.